Hf precursor TDEAH (Hf(NEt2)4): Difference between revisions
Jump to navigation
Jump to search
| Line 15: | Line 15: | ||
==Chemical reaction== | ==Chemical reaction== | ||
With a temperature of 117°C and a pressure of 0.04 torr the following reaction will occur within an inert atmosphere (Argon/Nitrogen): | |||
HfCl<sub>4</sub> + Et<sub>2</sub>NLi | HfCl<sub>4</sub> + Et<sub>2</sub>NLi | ||
→ | → | ||
TDEAH (Hf(NEt<sub>2</sub>)<sub>4</sub>) | TDEAH (Hf(NEt<sub>2</sub>)<sub>4</sub>) | ||
The yield typically is around 60% | |||
The waste products will be some carbon hydrates (not the stuff in white bread) | |||
Revision as of 13:51, 12 October 2022
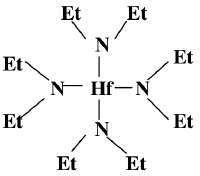
This is the structure of the organic precursor for Hafnium oxide deposition in a CVD (Hafnium_oxide_deposition_(CVD))
as described in the following Japanese paper
WARNING! This chemical reacts VIOLENTLY with water and humidity in general! Caution is required when dealing with it!
Atmospheric requirements
Since this mixture and its components react with oxygen and humidity, we need to use a Schlenk tube setup for mixing it, because we can only do it in an inert atmosphere.
Chemical reaction
With a temperature of 117°C and a pressure of 0.04 torr the following reaction will occur within an inert atmosphere (Argon/Nitrogen):
HfCl4 + Et2NLi → TDEAH (Hf(NEt2)4)
The yield typically is around 60%
The waste products will be some carbon hydrates (not the stuff in white bread)