Hf precursor TDEAH (Hf(NEt2)4): Difference between revisions
No edit summary |
|||
| (9 intermediate revisions by the same user not shown) | |||
| Line 10: | Line 10: | ||
Caution is required when dealing with it! | Caution is required when dealing with it! | ||
</span> | </span> | ||
===Chemical properties of Hafnium-Tetrachloride=== | |||
The base of this chemical recipe and processing is Hafnium-Tetrachloride, as it can be seen in the picture. | |||
[[File:High-Quality-Hafnium-Chloride-Hafnium-Tetrachloride-Hfcl4-CAS-No-13499-05-3-with-Best-Price.jpg|200px|Hafnium-Tetrachloride]] | |||
[[File:34591.png|200px|left]] | |||
HfCl<sub>4</sub> can be produced by several related procedures: | |||
*The reaction of carbon tetrachloride and hafnium oxide at above 450 °C; | |||
:HfO<sub>2</sub> + 2 CCl<sub>4</sub> → HfCl<sub>4</sub> + 2 COCl<sub>2</sub> | |||
*Chlorination of a mixture of HfO<sub>2</sub> and carbon above 600 °C using chlorine gas or sulfur monochloride: | |||
:HfO<sub>2</sub> + 2 Cl<sub>2</sub> + C → HfCl<sub>4</sub> + CO<sub>2</sub> | |||
*Chlorination of hafnium carbide above 250 °C. | |||
The result of those chemical reactions is a crystalline powder with a melting point of 432 °C | |||
You might notice that Hafnium-Tetrachloride is a solid crystal at room temperature, which is kind of a problem considering that we wanna use it as a vapor in our CVD furnace, in order to react it with oxide for obtaining a Hafnium-Oxide thin film layer. | |||
==Atmospheric requirements== | ==Atmospheric requirements== | ||
Since this mixture and its components react with oxygen and humidity, we need to use a Schlenk tube setup for mixing it, because we can only do it in an inert atmosphere. | Since this mixture and its components react with oxygen and humidity, we need to use a Schlenk tube setup for mixing it, because we can only do it in an inert atmosphere. | ||
==Chemical reaction== | |||
We combine lithium diethylamide (Et<sub>2</sub>NLi) with Hafnium tetrachloride (HfCl<sub>4</sub>), using a Schlenker tube setup. | |||
With a temperature of 117°C and a pressure of 0.04 torr the following reaction will occur within an inert atmosphere (Argon/Nitrogen): | |||
HfCl<sub>4</sub> + Et<sub>2</sub>NLi | |||
→ | |||
TDEAH (Hf(NEt<sub>2</sub>)<sub>4</sub>) | |||
The yield typically is around 60% | |||
The waste products will be some carbon hydrates (not the stuff in white bread) | |||
Latest revision as of 14:37, 12 October 2022
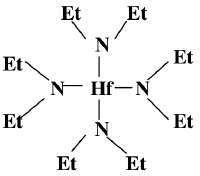
This is the structure of the organic precursor for Hafnium oxide deposition in a CVD (Hafnium_oxide_deposition_(CVD))
as described in the following Japanese paper
WARNING! This chemical reacts VIOLENTLY with water and humidity in general! Caution is required when dealing with it!
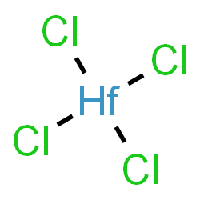
Chemical properties of Hafnium-Tetrachloride
The base of this chemical recipe and processing is Hafnium-Tetrachloride, as it can be seen in the picture.
HfCl4 can be produced by several related procedures:
- The reaction of carbon tetrachloride and hafnium oxide at above 450 °C;
- HfO2 + 2 CCl4 → HfCl4 + 2 COCl2
- Chlorination of a mixture of HfO2 and carbon above 600 °C using chlorine gas or sulfur monochloride:
- HfO2 + 2 Cl2 + C → HfCl4 + CO2
- Chlorination of hafnium carbide above 250 °C.
The result of those chemical reactions is a crystalline powder with a melting point of 432 °C
You might notice that Hafnium-Tetrachloride is a solid crystal at room temperature, which is kind of a problem considering that we wanna use it as a vapor in our CVD furnace, in order to react it with oxide for obtaining a Hafnium-Oxide thin film layer.
Atmospheric requirements
Since this mixture and its components react with oxygen and humidity, we need to use a Schlenk tube setup for mixing it, because we can only do it in an inert atmosphere.
Chemical reaction
We combine lithium diethylamide (Et2NLi) with Hafnium tetrachloride (HfCl4), using a Schlenker tube setup.
With a temperature of 117°C and a pressure of 0.04 torr the following reaction will occur within an inert atmosphere (Argon/Nitrogen):
HfCl4 + Et2NLi → TDEAH (Hf(NEt2)4)
The yield typically is around 60%
The waste products will be some carbon hydrates (not the stuff in white bread)