Hf precursor TDEAH (Hf(NEt2)4): Difference between revisions
No edit summary |
No edit summary |
||
| Line 1: | Line 1: | ||
The base of this chemical recipe and processing is Hafnium-Tetrachloride, as it can be seen in the picture. | The base of this chemical recipe and processing is Hafnium-Tetrachloride, as it can be seen in the picture. | ||
[[File:High-Quality-Hafnium-Chloride-Hafnium-Tetrachloride-Hfcl4-CAS-No-13499-05-3-with-Best-Price.jpg|200px|Hafnium-Tetrachloride]] | |||
===Chemical properties of Hafnium-Tetrachloride=== | ===Chemical properties of Hafnium-Tetrachloride=== | ||
| Line 15: | Line 17: | ||
The result of those chemical reactions is a crystalline powder with a melting point of 432 °C | The result of those chemical reactions is a crystalline powder with a melting point of 432 °C | ||
You might notice that Hafnium-Tetrachloride is a solid crystal at room temperature, which is kind of a problem considering that we wanna use it as a vapor in our CVD furnace, in order to react it with oxide for obtaining a Hafnium-Oxide thin film layer. | You might notice that Hafnium-Tetrachloride is a solid crystal at room temperature, which is kind of a problem considering that we wanna use it as a vapor in our CVD furnace, in order to react it with oxide for obtaining a Hafnium-Oxide thin film layer. | ||
Revision as of 14:20, 12 October 2022
The base of this chemical recipe and processing is Hafnium-Tetrachloride, as it can be seen in the picture.
Chemical properties of Hafnium-Tetrachloride
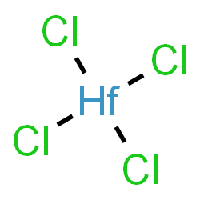
HfCl4 can be produced by several related procedures:
- The reaction of carbon tetrachloride and hafnium oxide at above 450 °C;
- HfO2 + 2 CCl4 → HfCl4 + 2 COCl2
- Chlorination of a mixture of HfO2 and carbon above 600 °C using chlorine gas or sulfur monochloride:
- HfO2 + 2 Cl2 + C → HfCl4 + CO2
- Chlorination of hafnium carbide above 250 °C.
The result of those chemical reactions is a crystalline powder with a melting point of 432 °C
You might notice that Hafnium-Tetrachloride is a solid crystal at room temperature, which is kind of a problem considering that we wanna use it as a vapor in our CVD furnace, in order to react it with oxide for obtaining a Hafnium-Oxide thin film layer.
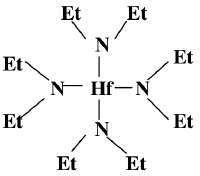
This is the structure of the organic precursor for Hafnium oxide deposition in a CVD (Hafnium_oxide_deposition_(CVD))
as described in the following Japanese paper
WARNING! This chemical reacts VIOLENTLY with water and humidity in general! Caution is required when dealing with it!
Atmospheric requirements
Since this mixture and its components react with oxygen and humidity, we need to use a Schlenk tube setup for mixing it, because we can only do it in an inert atmosphere.
Chemical reaction
With a temperature of 117°C and a pressure of 0.04 torr the following reaction will occur within an inert atmosphere (Argon/Nitrogen):
HfCl4 + Et2NLi → TDEAH (Hf(NEt2)4)
The yield typically is around 60%
The waste products will be some carbon hydrates (not the stuff in white bread)