Hafnium oxide deposition (CVD): Difference between revisions
(→Links) |
|||
| Line 23: | Line 23: | ||
===Processing steps=== | ===Processing steps=== | ||
Since HfCl<sub>4</sub> is a solid salt at room temperature, we | Since HfCl<sub>4</sub> is a solid salt at room temperature, we need to first create a liquid precursor, with a concentration range of 0.01-1.0M, and use [https://www.intechopen.com/chapters/63679 direct liquid injection] for using it in our CVD. | ||
The | The synthesis of this the [[Hf precursor TDEAH (Hf(NEt2)4)]] is so complex, that it needs its own page. | ||
===Links=== | ===Links=== | ||
Revision as of 12:42, 12 October 2022
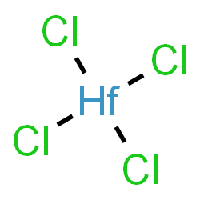
The base of this chemical recipe and processing is Hafnium-Tetrachloride, as it can be seen in the picture.
You might notice that Hafnium-Tetrachloride is a solid crystal at room temperature, which is kind of a problem considering that we wanna use it as a vapor in our CVD furnace, in order to react it with oxide for obtaining a Hafnium-Oxide thin film layer.
The equipment required for this process are a CVD and a plasma cleaner for removing impurities after the Hafnium oxide deposition
Chemical properties of Hafnium-Tetrachloride
HfCl4 can be produced by several related procedures:
- The reaction of carbon tetrachloride and hafnium oxide at above 450 °C;
- HfO2 + 2 CCl4 → HfCl4 + 2 COCl2
- Chlorination of a mixture of HfO2 and carbon above 600 °C using chlorine gas or sulfur monochloride:
- HfO2 + 2 Cl2 + C → HfCl4 + CO2
- Chlorination of hafnium carbide above 250 °C.
The result of those chemical reactions is a crystalline powder with a melting point of 432 °C
Processing steps
Since HfCl4 is a solid salt at room temperature, we need to first create a liquid precursor, with a concentration range of 0.01-1.0M, and use direct liquid injection for using it in our CVD.
The synthesis of this the Hf precursor TDEAH (Hf(NEt2)4) is so complex, that it needs its own page.
Links
Study HfO2 formation: https://iopscience.iop.org/article/10.1149/MA2005-02/13/547/pdf
A Japanese paper how they grew it with an LPCVD: http://www.trichemical.com/topics/Manuscript%20revisions.pdf