Hafnium oxide deposition (CVD): Difference between revisions
| Line 23: | Line 23: | ||
===Processing steps=== | ===Processing steps=== | ||
Since HfCl<sub>4</sub> is a solid salt at room temperature, we need to first create a liquid precursor | Since HfCl<sub>4</sub> is a solid salt at room temperature, we need to first create a liquid precursor, and use [https://www.intechopen.com/chapters/63679 direct liquid injection] for using it in our CVD. | ||
The process is based on a [https://download.libresilicon.com/papers/HafniumOxide.pdf Japanese paper] and requires a complex precursor. | |||
The synthesis of this the [[Hf precursor TDEAH (Hf(NEt2)4)]] is so complex, that it needs its own page. | The synthesis of this the [[Hf precursor TDEAH (Hf(NEt2)4)]] is so complex, that it needs its own page. | ||
Revision as of 12:47, 12 October 2022
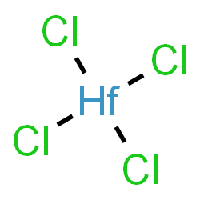
The base of this chemical recipe and processing is Hafnium-Tetrachloride, as it can be seen in the picture.
You might notice that Hafnium-Tetrachloride is a solid crystal at room temperature, which is kind of a problem considering that we wanna use it as a vapor in our CVD furnace, in order to react it with oxide for obtaining a Hafnium-Oxide thin film layer.
The equipment required for this process are a CVD and a plasma cleaner for removing impurities after the Hafnium oxide deposition
Chemical properties of Hafnium-Tetrachloride
HfCl4 can be produced by several related procedures:
- The reaction of carbon tetrachloride and hafnium oxide at above 450 °C;
- HfO2 + 2 CCl4 → HfCl4 + 2 COCl2
- Chlorination of a mixture of HfO2 and carbon above 600 °C using chlorine gas or sulfur monochloride:
- HfO2 + 2 Cl2 + C → HfCl4 + CO2
- Chlorination of hafnium carbide above 250 °C.
The result of those chemical reactions is a crystalline powder with a melting point of 432 °C
Processing steps
Since HfCl4 is a solid salt at room temperature, we need to first create a liquid precursor, and use direct liquid injection for using it in our CVD.
The process is based on a Japanese paper and requires a complex precursor.
The synthesis of this the Hf precursor TDEAH (Hf(NEt2)4) is so complex, that it needs its own page.
Links
Study HfO2 formation: https://iopscience.iop.org/article/10.1149/MA2005-02/13/547/pdf
A Japanese paper how they grew it with an LPCVD: http://www.trichemical.com/topics/Manuscript%20revisions.pdf