Hafnium oxide deposition (CVD): Difference between revisions
| (58 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
The | The equipment required for this process are a CVD and a plasma cleaner for removing impurities after the Hafnium oxide deposition | ||
Since HfCl<sub>4</sub> is a solid salt at room temperature, we need to first create a liquid precursor which can be turned into a vapor. | |||
The process is based on a [https://download.libresilicon.com/papers/HafniumOxide.pdf Japanese paper] and requires a complex precursor. | |||
The synthesis of the [[Hf precursor TDEAH (Hf(NEt2)4)]] is so complex, that it needs its own page. | |||
===Precursor injection=== | |||
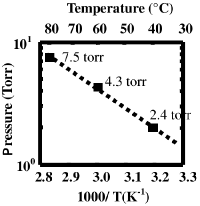
[[File:Pressure_TDEAH.png|200px|right|thumb|Vapor pressure]] | |||
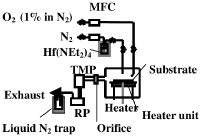
Introduce TDEAH gas into the deposition chamber using a bubbling system, use N<sub>2</sub> as the carrier gas. | |||
To prevent liquefaction of the source before it enters the deposition chamber, maintain the line from the bubbler to the chamber at a temperature of 85°C. | |||
https:// | Heat the bubbling system chamber to 80°C. | ||
The flow rate into the CVD chamber should be 40 sccm. | |||
===Oxidizer injection=== | |||
[[File:HFO2_CVD_setup.png|200px|right|thumb|CVD setup]] | |||
Have O<sub>2</sub> in N<sub>2</sub> in a ration 1:99 and feed it into the chamber through a separate nozzle, because otherwise the O<sub>2</sub> decomposes the TDEAH before it can reach the substrate. | |||
The flow rate can be varied between 0 and 20 sccm, for obvious reasons you want it higher than zero, because without oxygen you won't get any oxide. | |||
===Substrate/CVD chamber temperature=== | |||
Set a temperature between 300°C and 450°C, in order to control deposition speed. | |||
===CVD chamber pressure=== | |||
Set the pressure within the CVD chamber to 1 torr | |||
===Equipment=== | |||
* Potential CVD furnaces: | |||
** https://www.digiqualsystems.com/products/furnaces/tubular-furnace/horizontal-tubular-furnace/ | |||
** https://www.mtixtl.com/ThreeZonesTubeFurnaceHighVacuumMFCGas-OTF-1200X-III-HVC-UL.aspx | |||
* Bubblers: | |||
** https://www.mtixtl.com/Bubbler/Evaporatorforliquidsourcesandchemicalprecursors-BL-SS.aspx | |||
* Mass flow meters | |||
** https://www.mtixtl.com/Liquid-delivery-system.aspx | |||
Latest revision as of 17:33, 14 October 2022
The equipment required for this process are a CVD and a plasma cleaner for removing impurities after the Hafnium oxide deposition
Since HfCl4 is a solid salt at room temperature, we need to first create a liquid precursor which can be turned into a vapor.
The process is based on a Japanese paper and requires a complex precursor.
The synthesis of the Hf precursor TDEAH (Hf(NEt2)4) is so complex, that it needs its own page.
Precursor injection
Introduce TDEAH gas into the deposition chamber using a bubbling system, use N2 as the carrier gas.
To prevent liquefaction of the source before it enters the deposition chamber, maintain the line from the bubbler to the chamber at a temperature of 85°C.
Heat the bubbling system chamber to 80°C.
The flow rate into the CVD chamber should be 40 sccm.
Oxidizer injection
Have O2 in N2 in a ration 1:99 and feed it into the chamber through a separate nozzle, because otherwise the O2 decomposes the TDEAH before it can reach the substrate.
The flow rate can be varied between 0 and 20 sccm, for obvious reasons you want it higher than zero, because without oxygen you won't get any oxide.
Substrate/CVD chamber temperature
Set a temperature between 300°C and 450°C, in order to control deposition speed.
CVD chamber pressure
Set the pressure within the CVD chamber to 1 torr
Equipment
- Potential CVD furnaces:
- Mass flow meters