Hafnium oxide deposition (CVD): Difference between revisions
Jump to navigation
Jump to search
No edit summary |
|||
| Line 16: | Line 16: | ||
:HfO<sub>2</sub> + 2 Cl<sub>2</sub> + C → HfCl<sub>4</sub> + CO<sub>2</sub> | :HfO<sub>2</sub> + 2 Cl<sub>2</sub> + C → HfCl<sub>4</sub> + CO<sub>2</sub> | ||
*Chlorination of hafnium carbide above 250 °C. | *Chlorination of hafnium carbide above 250 °C. | ||
The result of those chemical reactions is a crystalline powder with a melting point of 432 °C | |||
===Processing steps=== | ===Processing steps=== | ||
https://patents.google.com/patent/CN100356519C/en | https://patents.google.com/patent/CN100356519C/en | ||
Revision as of 15:29, 11 October 2022
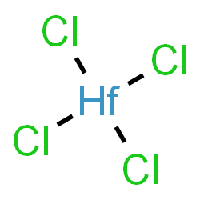
The base of this chemical recipe and processing is Hafnium-Tetrachloride, as it can be seen in the picture.
You might notice that Hafnium-Tetrachloride is a solid crystal at room temperature, which is kind of a problem considering that we wanna use it as a vapor in our CVD furnace, in order to react it with oxide for obtaining a Hafnium-Oxide thin film layer.
Chemical properties of Hafnium-Tetrachloride
HfCl4 can be produced by several related procedures:
- The reaction of carbon tetrachloride and hafnium oxide at above 450 °C;
- HfO2 + 2 CCl4 → HfCl4 + 2 COCl2
- Chlorination of a mixture of HfO2 and carbon above 600 °C using chlorine gas or sulfur monochloride:
- HfO2 + 2 Cl2 + C → HfCl4 + CO2
- Chlorination of hafnium carbide above 250 °C.
The result of those chemical reactions is a crystalline powder with a melting point of 432 °C